Reverse osmosis units
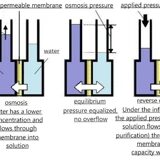
Reverse osmosis technology consists in the diffusion of liquid through semi-permeable membranes that allow the solvent (water) to pass through, but retain the dissolved substances (hydrated salt ions and organic molecules). Reverse osmosis is a baromembrane method in which the driving force is a pressure that exceeds the osmotic pressure.
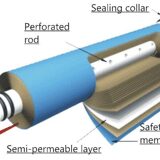
Water purification proceeds when filtering through one or more artificial porous membranes made of synthetic materials twisted into a roll.
The pore size in the membrane is 0.0001 microns. Accordingly only water molecules pass through the reverse osmosis membrane. The retention capacity of membrane elements for salts can reach up to 99.8%. It allows dividing the flow of incoming water into two. Permeate is purified water, which is further used for production needs. Concentrate - concentrated salt runoff discharged into the drainage system.
To prevent the precipitation of sparingly soluble compounds on the surface of the membranes, hydrochloric acid and a sedimentation inhibitor are dosed from the dosing station of reagents into the water flow supplied to the reverse osmosis unit in proportion to the flow rate in accordance with the dose set by the operator.
The reverse osmosis unit consists of several functional skids:
- Skid of preliminary fine cleaning on cartridge microfilters;
- Blocks of reagent processing;
- High pressure pumps;
- Hydraulic skid consisting of membrane modules;
- Pipe with fittings.
- Frame construction
- Automation, including executive and measuring device